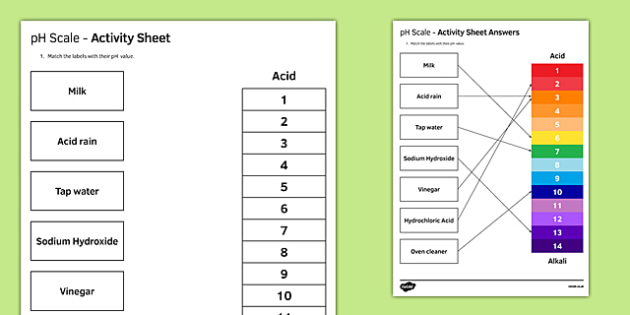
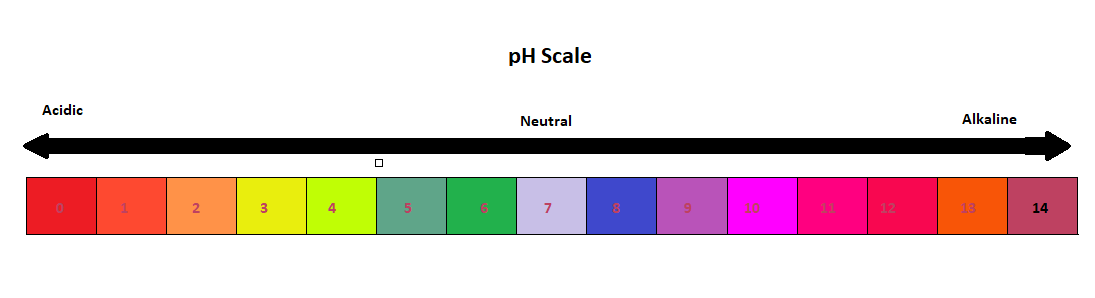
41 draw ph scale
3 Ways to Adjust Water pH - wikiHow Mar 31, 2022 · Most species of fish thrive in neutral water, so aim to keep your tank around a 7 on the pH scale. To adjust the pH in your swimming pool, use either sodium bisulfate or muriatic acid to keep it slightly basic, somewhere between 7.2 and 7.8 on the pH scale. Check your pool’s pH from time to time and lower it when needed, since the pH in pool ... Draw a pH scale and label water, hydrochloric acid, and sodi - quizlet.com Find step-by-step Biology solutions and your answer to the following textbook question: Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale..
Garden Map: How To Draw An Effective Annual Vegetable Garden ... Mar 13, 2022 · Step 2. Draw The Framework Onto Your Garden Layout Map. Once you’ve decided on a scale, it’s time to draw out the perimeters of your garden onto your map. Use a pen or dark color to draw the outside of your garden onto the mapping page (or graph paper). Write the length of each real side beside each side you draw.

Draw ph scale
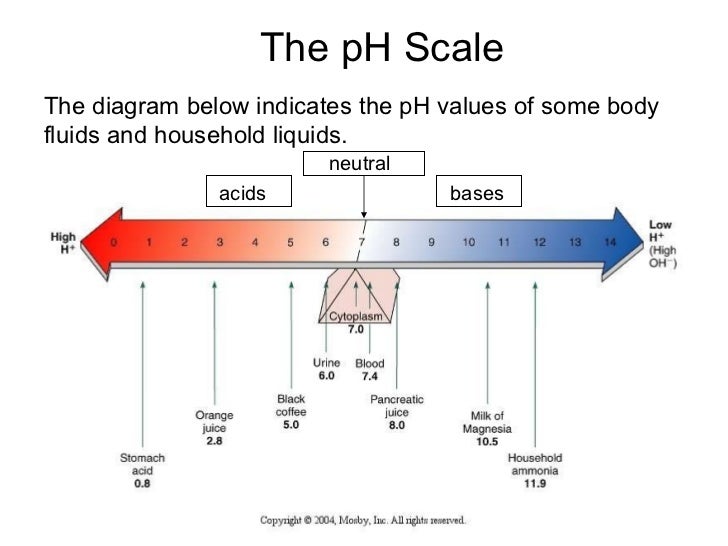
pH Scale - Acids and Bases A pH of 7 is neutral on the scale, greater than 7 is a base and less than 7 is an acid. Strong acids are mostly ranged at a pH of 0-2, strong bases have a range at a pH of 12-14. Colour Indicators. Colour Indicators are used to determine how acidic, basic or neutral the solution is. This method is told by the changing colour of the substance ... What Is the PH Scale and How Does It Work? - Reference.com The pH scale is a measure of how acidic or basic a liquid substance is. The pH is calculated as a negative logarithmic function of the concentration of hydrogen ions, which are acidic by nature. A high concentration of hydrogen ions will result in a low pH. The pH scale runs from 1.0 to 14.0. A substance can have a pH level of any finite number ... 12.7: The pH Scale - Chemistry LibreTexts pH is a logarithmic scale. A solution that has a pH of 1.0 has 10 times the [H +] as a solution with a pH of 2.0, which in turn has 10 times the [H +] as a solution with a pH of 3.0 and so forth. Using the definition of pH, it is also possible to calculate [H +] (and [OH − ]) from pH and vice versa.
Draw ph scale. pH Scale - pH | Dilution | Concentration - PhET Interactive Simulations Test the pH of things like coffee, spit, and soap to determine whether each is acidic, basic, or neutral. Visualize the relative number of hydroxide ions and hydronium ions in solution. Switch between logarithmic and linear scales. Investigate whether changing the volume or diluting with water affects the pH. Or you can design your own liquid! pH Scale: Acids, bases, pH and buffers (article) - Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens. Draw neat and labeled diagram of pH scale? - Toppr Ask Draw neat and labeled diagram of pH scale? Medium Solution Verified by Toppr The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 How do you draw the pH scale? - Answers The pH scale is often indicated as a vertical bar graph with scaled numbers from 0 to 14 (top to bottom). The lower numbers at the top are the more acidic pH, while the higher numbers near the...
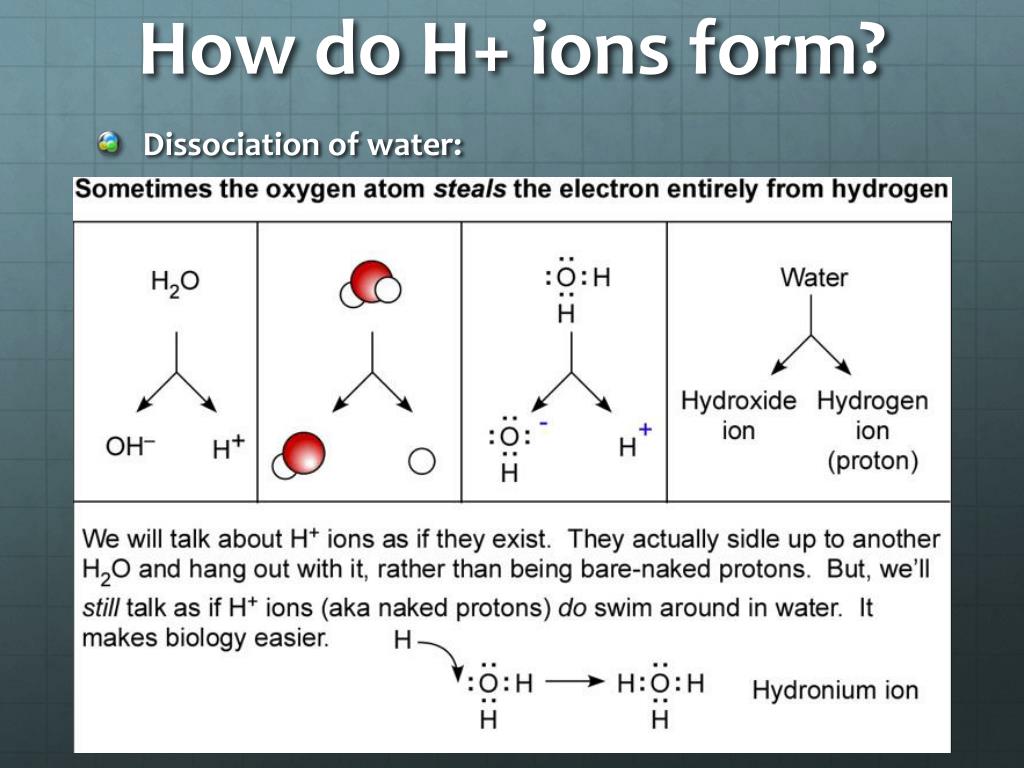
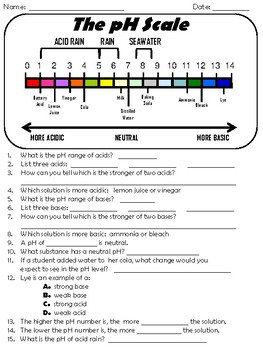
pH - Wikipedia In chemistry, pH (/ p iː ˈ eɪ tʃ /), historically denoting "potential of hydrogen" (or "power of hydrogen") is a scale used to specify the acidity or basicity of an aqueous solution.Acidic solutions (solutions with higher concentrations of H + ions) are measured to have lower pH values than basic or alkaline solutions.. The pH scale is logarithmic and inversely indicates the … Acids, Bases, & the pH Scale - Science Buddies In order to deal with these large numbers more easily, scientists use a logarithmic scale, the pH scale. Each one-unit change in the pH scale corresponds to a ten-fold change in hydrogen ion concentration. The pH scale is theoretically open-ended but most pH values are in the range from 0 to 14. The pH Scale | Biology for Non-Majors I | | Course Hero - Lumen Learning The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Most parts of our body (excluding things like stomach acid) measure around 7.2 and 7.6 on the pH scale (a 7 is neutral on the scale). If foreign strong substances dramatically change this pH, our bodies can no longer function properly. 12 draw the ph scale using numbers and label acid - Course Hero 12. Draw the pH scale using numbers and label acid, neutral, and base. (Using numbers 0-14) Acid AcidAcid Acid Acid AcidNeutral Base Base Base 10Base 11 Base 12 Base 13Base 141 23 4 5 67 8 9 Acid 1 Acid 2 Acid 3 Acid 4 Acid 5 Acid 6 Neutral 7 Base 8 Base 9 Base 10 Base 11 Base 12 Base 13 Base 14 13. What has more hydrogen ions, acids or bases? Base
pH Chemistry (Acids & Bases) - Definition, Calculating pH Value, Videos ... pH Chemistry. A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14: Litmus paper is an indicator used to tell if a substance is an acid or a base. The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. For example, Vinegar is an acid and measures 2.4 on ... pH Balance: How an Unbalanced pH Affects the Body 16/04/2018 · Restoring the pH balance in the body sometimes requires the body to draw from a stored base supply of carbonates and phosphates (usually in the bones), which can negatively affect proper functioning of these systems and structures. Acidosis, the presence of excess acid in the body’s fluids, is a major factor in stroke, diabetes, heart disease, osteoporosis, arthritis, … Baileigh Industrial - Metalworking & Woodworking Machinery Baileigh Industrial® Holdings LLC. distributes exclusively manufactured metal and woodworking machinery, trusted by customers ranging from large-scale commercial fabrication shops to passionate hobbyists. For 21 years, we’ve created machines that increase productivity, day in, day out, delivering repeatable quality results.. At Baileigh (pronounced “Bailey”), we build our … Solved 1. Draw the pH scale from 0 to 14. 2. Indicate where - chegg.com 1. Draw the pH scale from 0 to 14. 2. Indicate where on the scale it is acidic and basic and neutral. 3. At pH 3 and pH 12 value indicate the hydrogen ion concentration (For example at pH 7 hydrogen ion concentration is 0.0000001 M.) 4.
PDF pH Scale Activity - birdvilleschools.net On the construction paper, NEATLY draw a pH scale. 2. Scale the line from 0 to 14 with a mark for each number. 3. Cut out words & paste the labels in correct areas of pH scale. Weak Acid Strong Acid Strong Base Weak Base Neutral . 4. Color & Label the pH on the picture. Cut out & paste in the
Affinity – Professional Creative Software The fastest, smoothest and most precise image editing software around, this essential app will revolutionise the way you work, whether you’re editing and retouching images, creating full-blown multi-layered compositions or making beautiful raster paintings.
Answered: Describe or draw PH scale diagram and… | bartleby Describe or draw PH scale diagram and name one acid and base. Question. Describe or draw PH scale diagram and name one acid and base. Expert Solution. Want to see the full answer? Check out a sample Q&A here. See Solution. star_border. Students who've seen this question also like: BUY.
The pH scale - Acids, bases and salts - (CCEA) - BBC Bitesize The pH scale measures a solution's acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali). pH 0 - 2: strong acid pH 3 - 6: weak acid pH 7: neutral pH 8 ...
Molecule Shapes - VSEPR | Lone Pairs | Bonds - PhET Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!
pH Scale | U.S. Geological Survey The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic.
3 Ways to Adjust Water pH - wikiHow 31/03/2022 · Most species of fish thrive in neutral water, so aim to keep your tank around a 7 on the pH scale. To adjust the pH in your swimming pool, use either sodium bisulfate or muriatic acid to keep it slightly basic, somewhere between 7.2 and 7.8 on the pH scale. Check your pool’s pH from time to time and lower it when needed, since the pH in pool ...
pH Scale and Acidity - Properties and Limitaions of pH Scale - BYJUS pH = - log [H + ] p (OH) is the negative logarithm to the base ten of hydroxide ion concentration in moles per litre. p (OH) = - log [OH - ] In aqueous solutions, pH + p (OH) = 14. pH scale is based on neutral water, where [H +] = [OH -] = 10 -7 For a neutral solution pH = = - log [H +] = - log [10 -7] = +7
The pH Scale | Biology for Majors I | | Course Hero - Lumen Learning The pH is calculated as the negative of the base 10 logarithm of this concentration. The log10 of 1 × 10 -7 is -7.0, and the negative of this number (indicated by the "p" of "pH") yields a pH of 7.0, which is also known as neutral pH. The pH inside of human cells and blood are examples of two areas of the body where near-neutral pH is maintained.


Post a Comment for "41 draw ph scale"