44 olestra warning
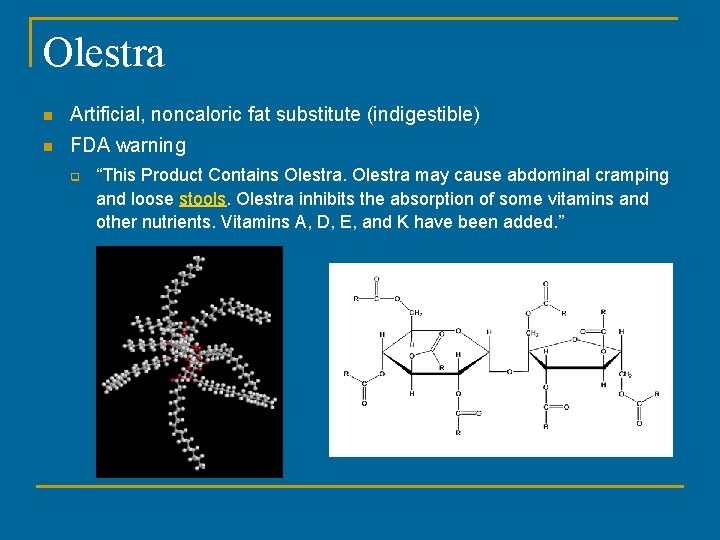
en.wikipedia.org › wiki › OlestraOlestra - Wikipedia Olestra uses sucrose as the backbone in place of glycerol, and it can form esters with up to eight fatty acids. Olestra is a mixture of hexa-, hepta-, and octa-esters of sucrose with various long chain fatty acids. The resulting radial arrangement is too large and irregular to move through the intestinal wall and be absorbed into the bloodstream. What Were They Thinking? The Chips That Sent Us Running To The Loo Warning signs are there for a reason. 2) Health Concerns -It's only human nature to want a "magic pill" so we can eat whatever we want without dietary consequences, but a diet full of olestra isn't...
Adverse Reactions to Olestra Have Nutrition Group Threatening to Sue ... The Center for Science in the Public Interest (CSPI), a Washington based nutrition group, is threatening litigation in Massachusetts against Frito-Lay for
Olestra warning
en.wikipedia.org › wiki › List_of_allergensList of allergens - Wikipedia Name Potential reaction(s) Remarks Balsam of Peru: Redness, swelling, itching, allergic contact dermatitis reactions, stomatitis (inflammation and soreness of the mouth or tongue), cheilitis (inflammation, rash, or painful erosion of the lips, oropharyngeal mucosa, or angles of their mouth), pruritus, hand eczema, generalized or resistant plantar dermatitis, rhinitis, conjunctivitis, and blisters. Olestra: A Leaky History - Portable Press Studies later indicated that Olestra's pants-ruining side effects were not as widespread as initially thought; i.e. not everyone who consumes the product suffers from uncontrollable diarrhea. That was good enough for the FDA, which no longer requires products made with Olestra to contain a warning label. Olestra To Lose Gastrointestinal Warning | American Council on Science ... Olestra To Lose Gastrointestinal Warning. Products containing Olestra, the zero-calorie fat substitute, will no longer bear a label informing consumers of purported unpleasant gastrointestinal (GI) side effects. The Food and Drug Administration (FDA), after reviewing a six-week study that involved 3000 people, ruled that Olestra "caused only ...
Olestra warning. en.wikipedia.org › wiki › SaccharinSaccharin - Wikipedia As a consequence, all food containing saccharin was labeled with a warning meeting the requirement of the Saccharin Study and Labeling Act of 1977. [32] However, in 2000, the warning labels were removed because scientists learned that rodents, unlike humans, have a unique combination of high pH, high calcium phosphate , and high protein levels ... › article › 440025-why-are-chipsThe Calories in Potato Chips, and 5 Reasons They're Unhealthy May 19, 2021 · Potato chips are among the most popular salted snacks in America. They're traditionally made out of thinly fried potatoes, but these days, it's easy to find alternative products like beetroot chips, corn chips or sweet potato chips. › en-us › health30 common U.S. foods that are banned in other countries - MSN Nov 27, 2021 · Products that contain Yellow 6 and Red 40 must include warning labels in the European Union. ... Olestra is a fat substitute the FDA approved in 1996 to make snacks and chips guilt-free. However ... Those gut-wrenching Olestra chips from the '90s might have ... - Quartz It was also a massive pain—in the gastrointestinal area, to be precise. It became notorious for its warning of "abdominal cramping and loose stools." But a new study has found that Olestra might...
Olestra: The Embarrassing Story Behind the Once-Famous Fat ... - Cookist A little too quick. Customers who consumed these fat-free snacks, complained of stomach cramps, diarrhea, and the most embarrassing… anal leakage. So the FDA later required all products containing olestra to be labelled with a warning of the potential side effects. The Potato Chip That Destroyed The Bowels Of America The fact that Olestra products were required to have a big "Warning: Your Ass May Turn Into The Bellagio Fountains At Any Moment" label on every bag caused customers to associate any intestinal discomfort with Olestra, whether it was caused by the product or not. FOOD INDUSTRY Those Olestra warnings come and go When does a potential health effect become significant enough to require a warning, and when might the warning itself create a problem? How olestra works Olestra, a zero-calorie soybean-and-sugar concoction three decades in the making, tastes like real fat and has a similar feel in the mouth, but passes out of the body undigested. Developed and ... Olestra warning!! - YouTube ignorejust uploaded for a chem. project..
Olestra Fat-Free Snack Controversy of the 1990s | Mental Floss The agency also mandated a package warning about abdominal cramping and loose stools, an observed side effect of olestra consumption. Procter & Gamble made a minor stir about the label—after all,... Olestra Side Effects - Nutrineat Gastrointestinal Problems: Over consumption of products that contain this addictive can lead to many gastrointestinal issues, like abdominal cramps, diarrhea, and loose stools (this condition is also known as anal leakage). It may also lead to fatigue and nausea, followed by vomiting. FDA removes Olestra warning - NBC News Snacks made with the fake fat olestra no longer will have to bear the unappetizing label that warned they might cause cramps and diarrhea. The Food and Drug Administration lifted the warning... FDA Eliminates Digestive Warning From Olestra | Progressive Grocer Studies using normal consumption of products with olestra showed the additive caused only infrequent, mild gastrointestinal effects, the FDA said. Before Friday's ruling, packages of products containing Olestra had to contain a label that read: "This product contains olestra. Olestra may cause abdominal cramping and loose stools.
FDA Memo Concluded Olestra Causes Diarrhea | Center for Science in the ... These conclusions reinforce the need to have a clear, informative label on all olestra-containing products." In 1996, the FDA approved the use of olestra in salty snack foods. Currently, the FDA is considering whether to drop or retain the olestra warning notice ("may cause abdominal cramping or loose stools") from packages.
FDA panel generally endorses safety of olestra - June 17, 1998 - CNN FDA panel generally endorses safety of olestra But product warning labels to remain. In this story: ... Olean - Procter and Gamble's official Olestra site Center for Science in the Public Interest.
Olestra Warning No Longer Required\ the Fda Says the Fat Substitute ... Snacks made with the fake fat olestra no longer must bear the unappetizing label that warned they might cause cramps and diarrhea.
› story › 5-sneaky-ingredients-in-food5 Sneaky Ingredients In Food That Can Cause Diarrhea | SELF Apr 08, 2016 · The FDA used to require a warning label on any olestra-containing products, but has since lifted the requirement. Many products containing olestra have been discontinued, like Lay's Wow potato ...
PDF Re: The Urgent Need for Warning Label on Synthetically Dyed Foods to ... In regards to olestra, the FDA's principal concern in requiring a warning label was not related to the severity of the symptoms caused by its ingestion. In fact, the FDA repeatedly stated that it found "no safety concerns with respect to the effect of olestra on the gastro-intestinal (GI) tract." 9. FDA stated:
en.wikipedia.org › wiki › SteatorrheaSteatorrhea - Wikipedia As a result, the product was reformulated before general release to a hydrogenated form that is not liquid at physiologic temperature. The U.S. Food and Drug Administration warning indicated excessive consumption of Olestra could result in "loose stools"; however, this warning has not been required since 2003. Diagnosis
'Wow! These Chips Just Made Me Crap My Pants': The Olestra Story Nearly 500,000 samples of olestra-fried, fat-free versions of Pringles, Lays, Ruffles and Doritos were handed out, and the response was largely receptive. Receptive enough, in fact, that consumers were willing to overlook the required warning label that read, "Olestra may cause abdominal cramping and loose stools." Recommended Reading
Olestra - Cnn April 10 -- Health Briefs: Olestra makers ask FDA to tone down warning labels; July 2 -- Health Briefs: Fat substitute may be bad for health August 25 -- New fake fat: tastes good, less filling
Olestra To Lose Gastrointestinal Warning | American Council on Science ... Olestra To Lose Gastrointestinal Warning. Products containing Olestra, the zero-calorie fat substitute, will no longer bear a label informing consumers of purported unpleasant gastrointestinal (GI) side effects. The Food and Drug Administration (FDA), after reviewing a six-week study that involved 3000 people, ruled that Olestra "caused only ...
Olestra: A Leaky History - Portable Press Studies later indicated that Olestra's pants-ruining side effects were not as widespread as initially thought; i.e. not everyone who consumes the product suffers from uncontrollable diarrhea. That was good enough for the FDA, which no longer requires products made with Olestra to contain a warning label.
en.wikipedia.org › wiki › List_of_allergensList of allergens - Wikipedia Name Potential reaction(s) Remarks Balsam of Peru: Redness, swelling, itching, allergic contact dermatitis reactions, stomatitis (inflammation and soreness of the mouth or tongue), cheilitis (inflammation, rash, or painful erosion of the lips, oropharyngeal mucosa, or angles of their mouth), pruritus, hand eczema, generalized or resistant plantar dermatitis, rhinitis, conjunctivitis, and blisters.

































Post a Comment for "44 olestra warning"