40 multistep reaction energy profile
PDF Unit 5 - Kinetics of a chemical reaction on temperature can be expressed by the following equation, known as the Arrhenius equation k = Ae -E a /RT here, w A = collision frequency (can be considered a constant) E a = activation energy R = the gas constant (8.314 J K -1 mol -1 ) T = absolute temperature (in K) Taking ln Reaction mechanism and rate law (article) | Khan Academy Summary. A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step.
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide Energy profiles for reactions which go via a single transition state only This is much easier to talk about with a real example. The equation below shows an organic chemistry reaction in which a bromine atom is being replaced by an OH group in an organic compound. The starting compound is bromoethane, and the organic product is ethanol.
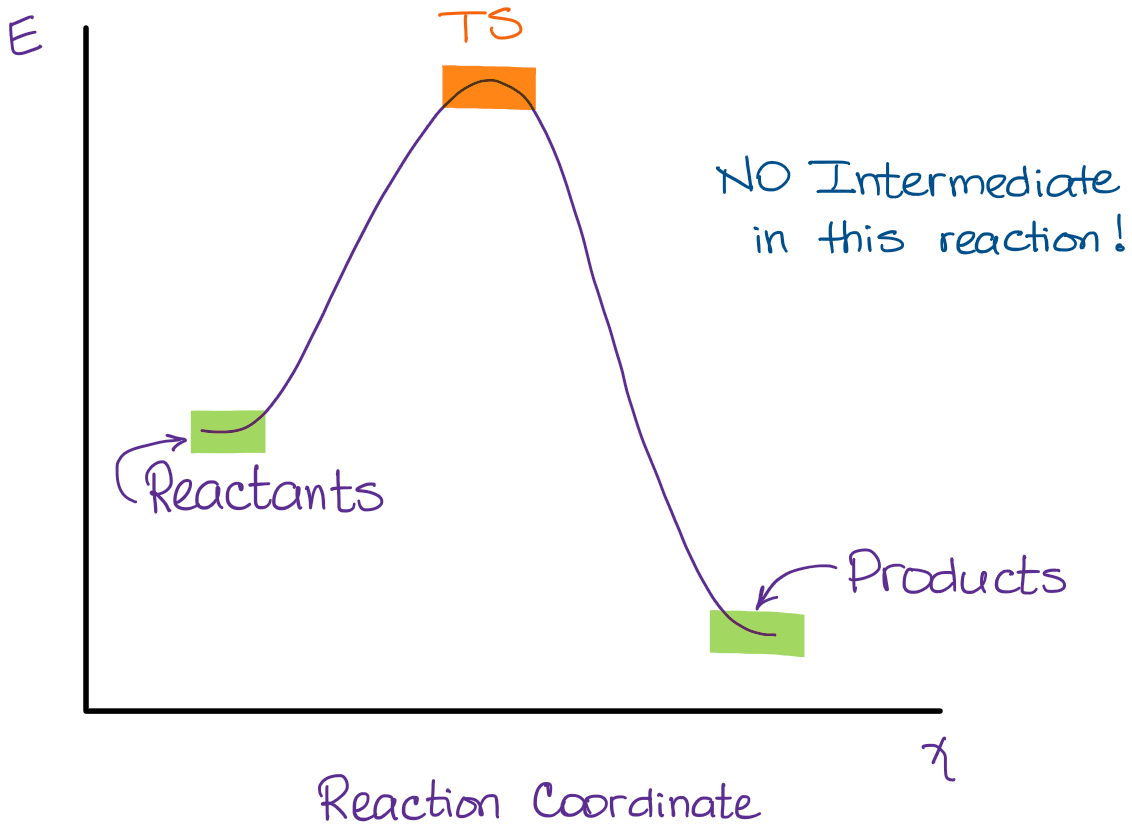
Multistep reaction energy profile
PDF Multi-Step Energy Calculations, Extra Exercises 2. How much energy is released when 1.00 t of sulfur trioxide is produced by the following reaction? 2 SO 2(g) O 2(g) → 2 SO 3(g) ∆H -192.8 kJ 3. In respiration, glucose is oxidized by oxygen gas to produce carbon dioxide gas, liquid water, and energy. What is the energy released when 18.0 g of glucose is consumed? 4. Methanol is burned ... autodE: Automated Calculation of Reaction Energy Profiles— Application ... Calculating reaction energy profiles to aid in mechanistic elucidation has long been the domain of the expert computational chemist. ... The general applicability of autodE is demonstrated in complex multi-step reactions, including cobalt- and rhodium-catalyzed hydroformylation and an Ireland-Claisen rearrangement. Single-atom catalysis of CO oxidation using Pt1/FeOx - Nature Jul 22, 2011 · For CO oxidation, sample A gave a specific reaction rate of 0.435 mol CO h −1 g Pt −1 at the reaction temperature of 27 °C, which is double that of Au/Fe 2 O 3 and almost triple that of sample B.
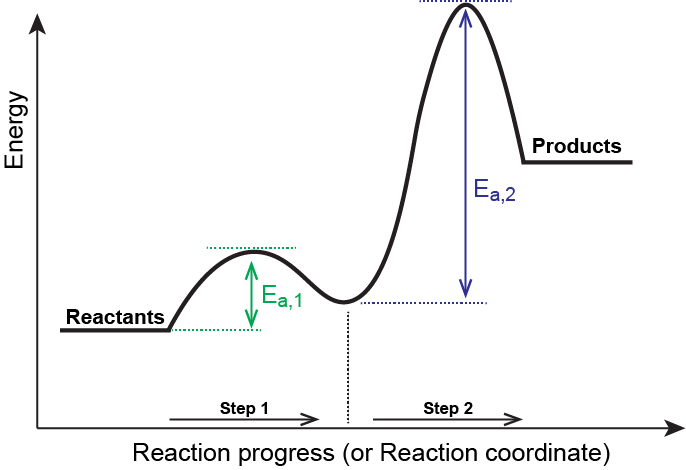
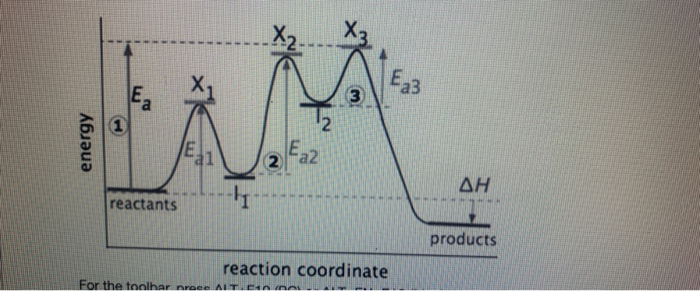
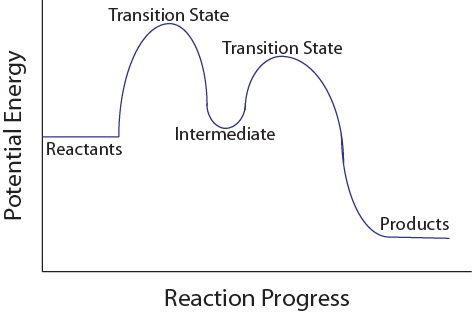
Multistep reaction energy profile. 5.10+Multistep+Reaction+Profile+Diagram+Student.pdf - 5.10... NOTES: Reaction energy profiles can also be used to illustrate a multistep reaction, as long as the energetics for each step are known. In a two-step reaction, two transition states are shown. In a three-step mechanism, three transitionstates are shown. Exploring Reaction Energy Profiles Using the Molecules-in-Molecules ... to study the potential energy profiles for multistep chemical reactions using the MIM methodology. In a complex multistep chemical reaction, the fragmentation scheme needs to be changed as the reacting species transition into a new reaction step, resulting in a discontinuity in the potential energy curve of the reaction. In our approach, the Analyzing Multi-step Reaction Energy Profiles | Chemistry | Study.com In a reaction energy profile, this is shown as everything between two low points. Intermediate: In a multi-step reaction, this is a relatively stable or low energy state and is shown by a low point... Reaction mechanism - Wikipedia The electron or arrow pushing method is often used in illustrating a reaction mechanism; for example, see the illustration of the mechanism for benzoin condensation in the following examples section.. A reaction mechanism must also account for the order in which molecules react. Often what appears to be a single-step conversion is in fact a multistep reaction.
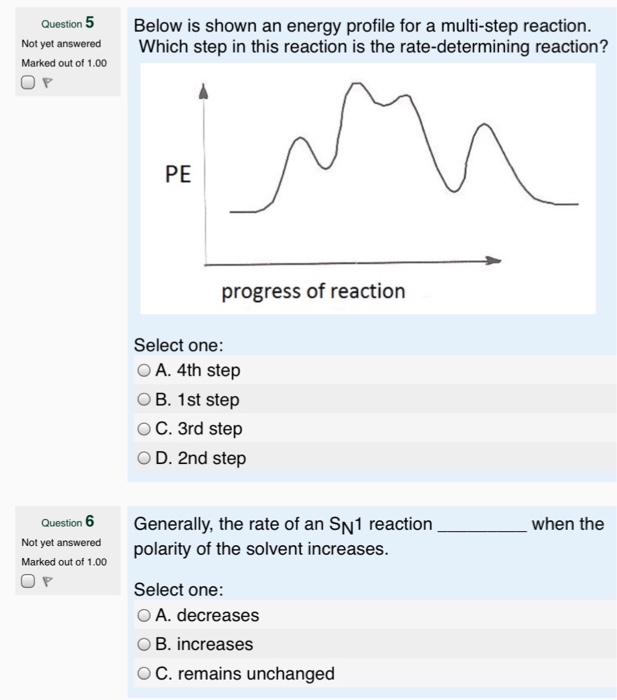
Publications – Pradeep Research Group New routes for multi-component atomically precise metal nanoclusters, Esma Khatun, and Thalappil Pradeep, ACS Omega, 6 (2021) 1-16 (DOI: 10.1021/acsomega.0c04832). (Invited Perspective) PDF File Differential risk factor profile of diabetes and atherosclerosis in rural, sub-urban and urban regions of South India: The KMCH-Non-communicable disease studies, … PDF Unit 5 - Kinetics - Chemistry Teaching Resources The previous diagram shows a typical reaction pathway for a multi-step reaction, in which the first stepis the slowest step(highest activation energy). The diagram below illustrates the situation where the slowest step is not the first step. AP e e a 2021 page 73 e 1.hen free ClW (g) atoms encounter O 3(g) 5.10 - Multistep Reaction Energy Profiles - YouTube Notes graphic organizer can be found at: video reviews how a reaction ... Exploring Reaction Energy Profiles Using the Molecules-in-Molecules ... The present work delineates a protocol to study the potential energy profiles for multistep chemical reactions using the MIM methodology. In a complex multistep chemical reaction, the fragmentation scheme needs to be changed as the reacting species transition into a new reaction step, resulting in a discontinuity in the potential energy curve ...
AP Chemistry – AP Students | College Board Multistep reaction energy profile; Catalysis; On The Exam. 7%–9% of exam score . Unit 6: Thermodynamics You’ll learn about energy changes in chemical reactions and how a transfer of energy can change a substance’s physical qualities. Topics may include: Endothermic and exothermic processes ; Heat transfer and thermal equilibrium; Heat capacity and calorimetry; … American Chemical Society Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. Progress and Perspectives of Electrochemical CO2 Reduction on … There is a pressing need to advance the development of CO 2 utilization technologies such as electrochemical CO 2 reduction (CO 2 R); as a result, this has been a rapidly expanding field of research in recent years. In particular, there is a large body of work on copper (Cu) materials for this reaction, since Cu is, as of yet, unique in its ability to catalyze the electrochemical … Collision theory and the Maxwell-Boltzmann distribution - Khan Academy Collision theory says that particles must collide in the proper orientation and with enough kinetic energy to overcome the activation energy barrier. So let's look at the reaction where A reacts with B and C to form AB plus C. On an energy profile, we have the reactants over here in the left. So A, atom A is colored red, and we have molecule BC ...
Multistep Reaction Energy Profile | StudyAPChemistry This is an exothermic reaction, as heat energy is released. We will cover this more in the next unit, which is Thermodynamics. That's basically it for the Multistep Reaction Energy Profile. If you are interested in moving onto Catalysts, which plays a big part in Kinetics, please take a look at the next guide, which is the final one for this unit.
Unit 5.10 - Multistep Reaction Energy Profiles - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
5 10 multistep reaction energy profile - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
5.10 Multistep reaction energy profiles.pdf - Course Hero 5.10 Multistep reaction energy profile Review: Most reactions have multiple steps The rate of reaction is determined by the slowest step In a single step reaction, you see a simple energy diagram like the one on the right: In multi-step reactions, each elementary step has its own activated complex and its own activation energy barrier. The slow step has an activation energy barrier that is ...

How to Draw Multi-Steps Energy Profile Diagrams: Reactant, Product, ∆H, Activation Energy, Slow Step
USATestprep: K-12 standards-aligned practice tests (5.6) Reaction Energy Profile (5.7) Introduction to Reaction Mechanisms (5.8) Reaction Mechanism and Rate Law (5.9) Steady-State Approximation (5.10) Multistep Reaction Energy Profile (5.11) Catalysis; Thermodynamics (6.1) Endothermic and Exothermic Processes (6.2) Energy Diagrams (6.3) Heat Transfer and Thermal Equilibrium
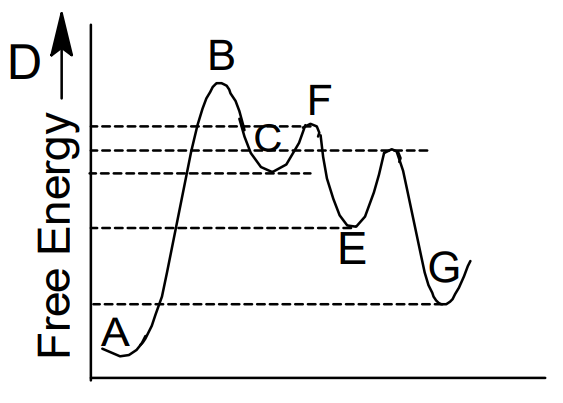
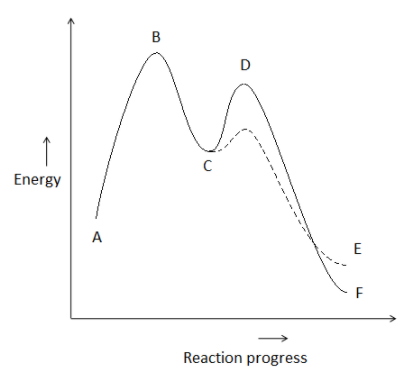
Solved The energy profile diagram below of a multi-step - Chegg The energy profile diagram below of a multi-step reaction Examine the diagram and answer the following questions B Gibbs Energy M У А G Reaction progress I. How many steps are there in this reaction? Less Is the first step in the reaction endergonic or exergonic?
PDF MULTISTEP REACTIONS - msu.ru MULTISTEP REACTIONS 1. INTRODUCTION ТЬе DWA as used in Chapter V and Section VI.3 describes а single-step reaction as exhibited Ьу the explicit арреагапсе of the responsible interaction only опсе in the transition matrix element [see (У.4.8) and (VI.2.26')]. Моге picturesquely, опе visualizes the incident projectile passing пеаг
Pediatric Feeding and Swallowing - American Speech-Language ... According to the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; American Psychiatric Association, 2016), ARFID is an eating or a feeding disturbance (e.g., apparent lack of interest in eating or in food, avoidance based on the sensory characteristics of food, concern about aversive consequences of eating), as manifested by ...
Lesson Explainer: Reaction Profiles | Nagwa The reaction of hydrogen gas with oxygen gas to produce water is an exothermic reaction, producing 285.8 kJ/mol of energy per mole of hydrogen gas. The chemical equation for this reaction can be written as H () + O () H O () k J m o l 2 2 2 g g l 1 2 + 2 8 5. 8 / Using this information, the following energy level diagram can be constructed.
Chapter 19 Chem 2 Flashcards | Quizlet Correctly order the steps required to derive the overall rate law for a multistep reaction that has one or more fast initial steps. 1. Express the equation of each fast step preceding the rate-determine step as an equilibrium process ... There will be three peaks in the energy profile The energy of the products will be lower than the energy of ...
Lanthanide-based hybrid nanostructures: Classification, synthesis ... 1. Introduction. Recently, the development of noble and efficient optical materials has made a great impact in the area of device development , , , .In this context, interest in lanthanides, as an activator, has grown enormously because of their excellent optical parameters such as sharp emission line casing ultraviolet-visible-infrared spectral region, long-lived and dense energy …
A Guide to AP® Chemistry Units & Topics - UWorld College Prep 5.10 Multistep Reaction Energy Profile: Learn to depict the reaction energy profiles of multistep reactions, showing the activation energy and overall energy change of the reaction. 3. Representing Data and Phenomena: 5.11 Catalysis: Learn how catalysts affect reactions by altering activation energy and/or reaction mechanisms. 6. Argumentation
Topic 5.10 (Answer Key) Multistep Reaction Energy Profile.docx The student collects the H2(g) produced by the reaction and measures its volume over water at 298 K after carefully equalizing the water levels inside and outside the gas-collection tube, as Q&A On the incomplete reaction energy diagram below, draw a curve that shows the following two details.
Answered: A reaction is Oth order in reagent B.… | bartleby The reaction profile shown to the right represents energy changes as a reaction proceeds. a) How… A: The reaction profile diagram of the reaction shows the potential energy of the reactants and the… Q: Consider the multistep reaction. What is the best rate law for the overall reaction? A) Rate =… A: Click to see the answer. Q: 23. A reaction proceeds by a series of elementary steps …
Multistep reaction energy profiles | Kinetics | AP Chemistry | Khan ... Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the act...
Multistep reaction energy profiles (video) | Khan Academy Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction.
Analyzing Multi-step Reaction Energy Profiles - Study.com Analyzing Multi-step Reaction Energy Profiles High School Chemistry Skills Practice 1. Considering the graph, how many elementary steps are in the reaction mechanism? 2. On the following multi-step...
Energy Diagram Module Series- Part Three: Intermediates and Rate ... Posted on August 12th, 2013. This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism.
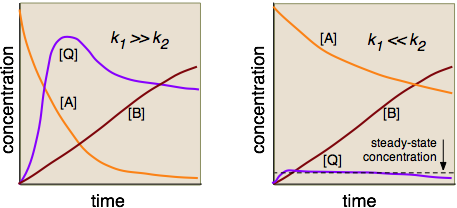
Multistep Reactions - Softschools.com The rate of a multistep reaction depends on what species are involved before the slowest (rate-determining) step. Species can be formed, then consumed, in the reaction, but do not exist for a long period of time and do not appear in the overall reaction equation. These transient species are called intermediates.
Single-atom catalysis of CO oxidation using Pt1/FeOx - Nature Jul 22, 2011 · For CO oxidation, sample A gave a specific reaction rate of 0.435 mol CO h −1 g Pt −1 at the reaction temperature of 27 °C, which is double that of Au/Fe 2 O 3 and almost triple that of sample B.
autodE: Automated Calculation of Reaction Energy Profiles— Application ... Calculating reaction energy profiles to aid in mechanistic elucidation has long been the domain of the expert computational chemist. ... The general applicability of autodE is demonstrated in complex multi-step reactions, including cobalt- and rhodium-catalyzed hydroformylation and an Ireland-Claisen rearrangement.
PDF Multi-Step Energy Calculations, Extra Exercises 2. How much energy is released when 1.00 t of sulfur trioxide is produced by the following reaction? 2 SO 2(g) O 2(g) → 2 SO 3(g) ∆H -192.8 kJ 3. In respiration, glucose is oxidized by oxygen gas to produce carbon dioxide gas, liquid water, and energy. What is the energy released when 18.0 g of glucose is consumed? 4. Methanol is burned ...






















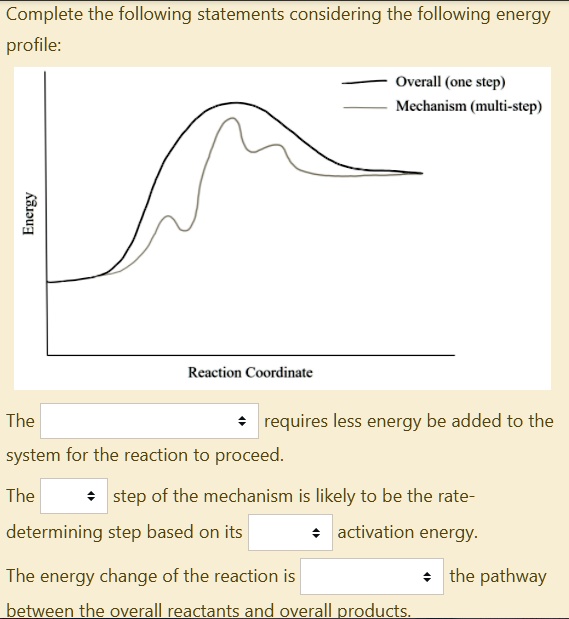
Post a Comment for "40 multistep reaction energy profile"