44 multi step reaction energy diagram
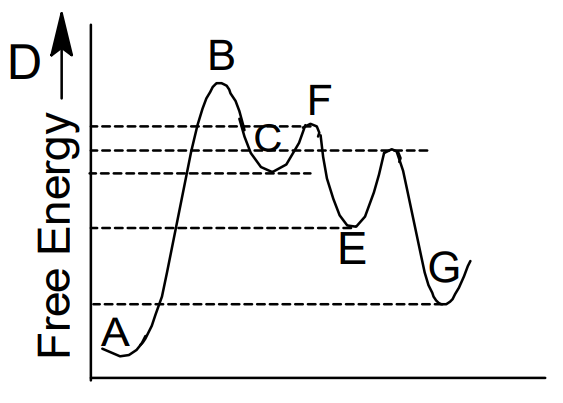
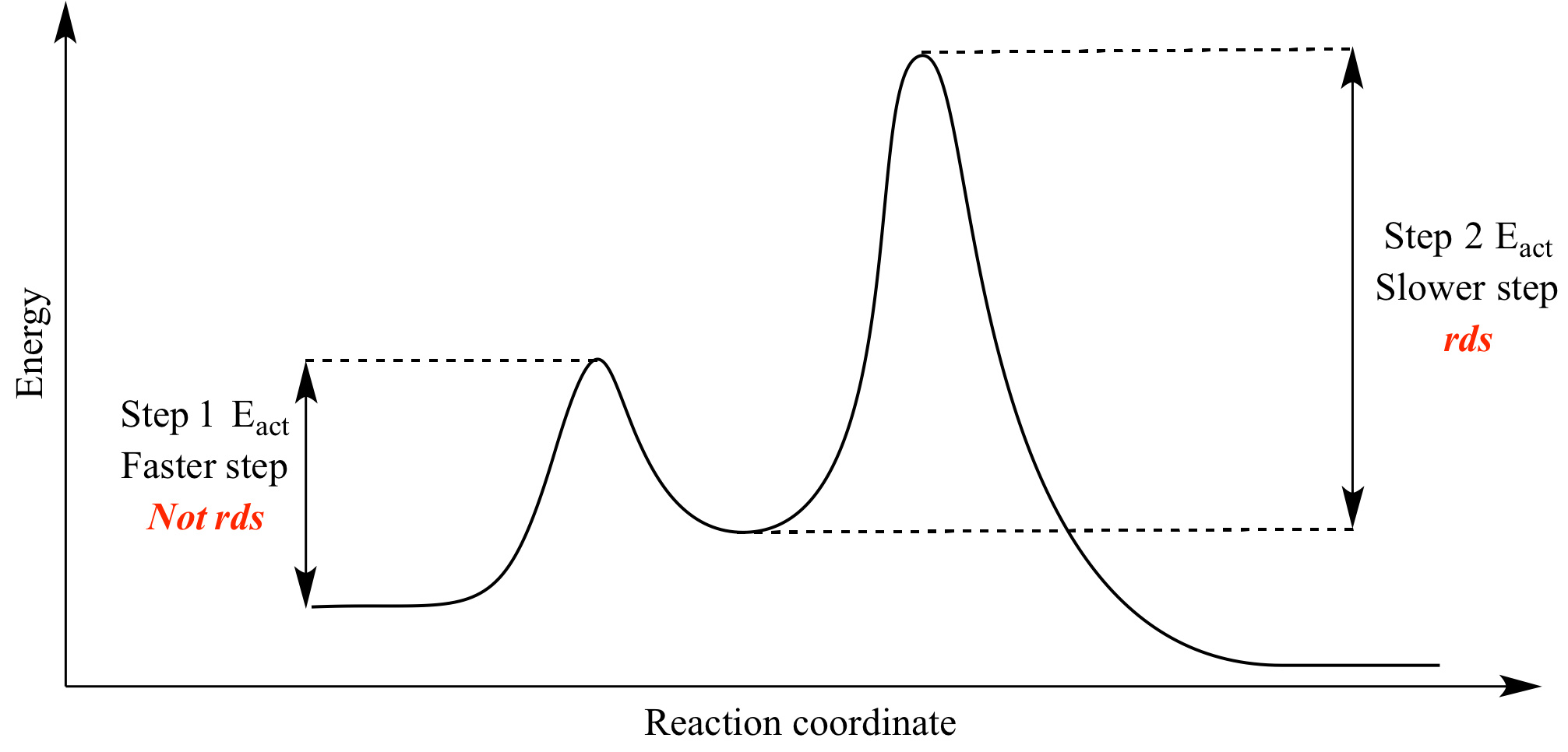
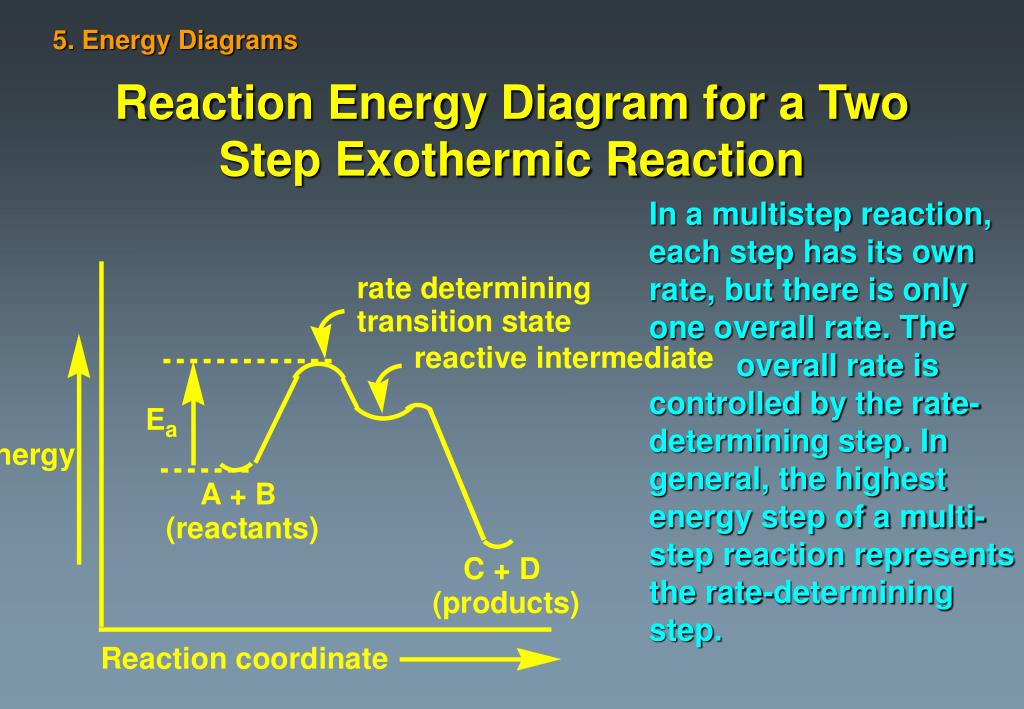
Does the rate determining step have the highest activation energy ... Answer: Not necessarily. In a multi-step mechanism, the reaction coordinate diagram has a lot of peaks and valleys, but the rate determining step is the last one that brings you to the top of the mountain. Often the activation energy for this step is much smaller than the one(s) that precede it. ... Reaction Profiles; Rate Limiting Steps – Chem 103/104 Resource ... In a reaction coordinate diagram, each step in a multi-step mechanism will have its own activation energy, resulting in an energy peak for each step.
TIGER - NCSSM Distance Education and Extended Programs This diagram shows a potential energy diagram for a multi-step mechanism. NOplusH2_ver_1-640.gif This diagram shows a one-step mechanism for a reaction. NOplusH2_ver_2-640.gif This diagram shows a two-step mechanism for a reaction with the first step being rate determining. NOplusH2_ver_3-640.gif This diagram shows a two-step mechanism for a ...
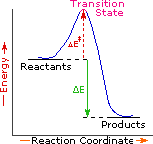
Multi step reaction energy diagram
Reaction mechanism and rate law (article) | Khan Academy A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step. Enzyme kinetics - Wikipedia Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions.In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier … 5.10 - Multistep Reaction Energy Profile - YouTube Nov 20, 2020 ... 5.10 - Multistep Reaction Energy Profile. Watch later. Share. Copy link. Info. Shopping. Tap to unmute. If playback doesn't begin shortly, ...
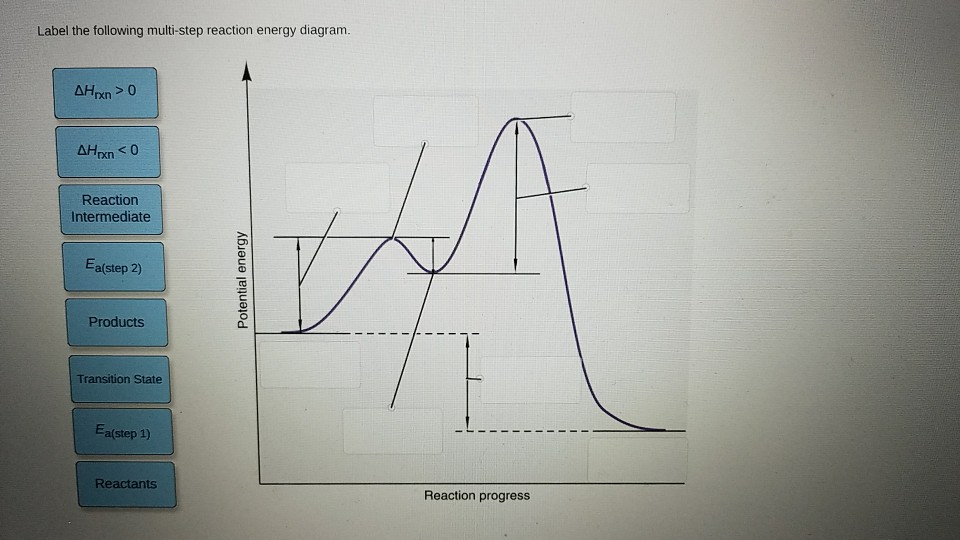
Multi step reaction energy diagram. Label the following multi-step reaction energy diagram. - Chegg Expert Answer. 99% (128 ratings) Transcribed image text: Label the following multi-step reaction energy diagram. Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy levels is as follows: Illustrating the overall reaction network of the synthesis-gas-to ... 15/09/2022 · Typically, Fe-, Co-, Ni- and Ru-based materials are active FTS catalysts. 2, 3, 5 Owing to its compatibility for the water-gas shift reaction (WGSR: CO + H 2 O ⇌ H 2 + CO 2), iron-based FTS catalysts are currently the most pragmatic option, especially for feedstocks typically with a low H 2 /CO ratio (also an inexpensive and abundant option than other metals). … Reaction Mechanisms - Chem1 2 Multi-step (consecutive) reactions. Mechanisms in which one elementary step is followed by another are very common. step 1: A + B → Q. step 2: B + Q → C. net reaction: A + 2B → C. (As must always be the case, the net reaction is just the sum of its elementary steps.) In this example, the species Q is an intermediate, usually an unstable ...
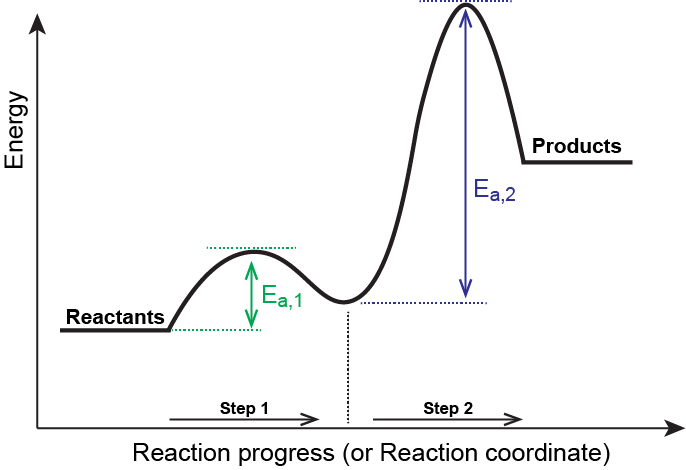
Reaction Mechanisms – Introductory Chemistry – 1st Canadian Edition To become familiar with reaction potential energy diagrams. To gain an understanding of rate-determining steps in multi-step reactions. 1.5.2 Energy Level Diagrams - Save My Exams Step 1: The chemical equation for the complete combustion of methane is: Step 2: Combustion reactions are always exothermic (Δ H is negative) so the reactants should be drawn higher in energy than the products. Step 3: Draw the curve in the energy level diagram clearly showing the transition state. Step 4: Draw arrows to show the Ea and Δ H ... Multistep reaction energy profiles (video) | Khan Academy Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction. 18.5 Multi step Reaction - CK-12 Feb 23, 2012 ... When there is a single step reaction, we can draw potential energy diagrams like the ones we have seen earlier in this chapter. For a multi-step ...
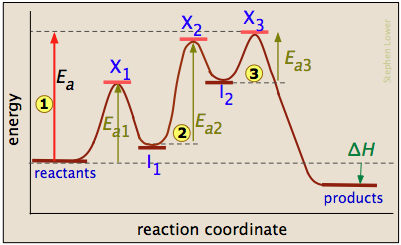
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. Multi-Step Reaction - an overview | ScienceDirect Topics The multi-step reaction catalyzed by the PDH complex involves sequential reactions with the intermediates being relayed between the subunits via the flexible lipoyl domains. E1 catalyzes the decarboxylation of pyruvate forming 2- (1-hydroxyethyldiene)-TPP bound to E1 (TPP is thiamin pyrophosphate). Ch 16 Pt 2 Smartbook Flashcards | Quizlet An elementary step is characterized by its _____, which is equal to the number of reactant particles in the step. Multiple choice question. molecularity. ... For the reaction represented by the accompanying reaction energy diagram, the _____ step is _____ and is rate limiting. first, slower. For any mechanism, only _____, involved up to and ... Reaction Rates & Why Reactions Take Time - 800mainstreet.com In this illustration the blue curve shows the reaction energy diagram for an exothermic reaction. The products have lower potential energy than the reactants. The red curve shows what a catalyst does to the activation energy and reaction path. ... It is a multi step reaction . Most reactions occur through a series of steps called a mechanism ...
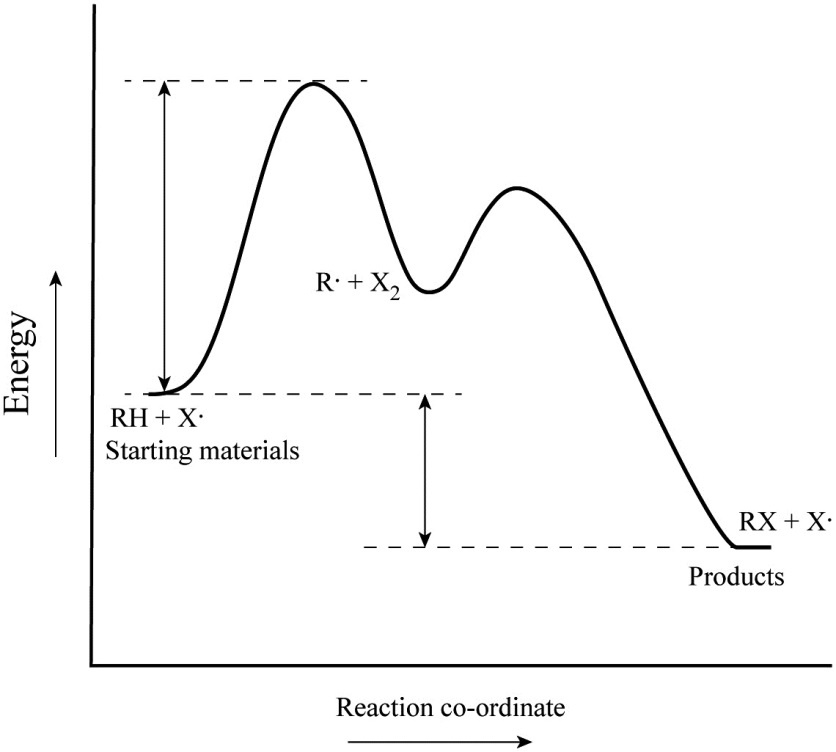
Rate-determining step - Wikipedia A multistep example is the reaction between oxalic acid and chlorine in aqueous solution: H 2C 2O 4 + Cl 2 → 2 CO 2 + 2 H+ + 2 Cl− . [7] The observed rate law is which implies an activated complex in which the reactants lose 2 H+ + Cl− before the rate-determining step. The formula of the activated complex is Cl 2 + H 2C 2O 4 − 2 H+ − Cl−

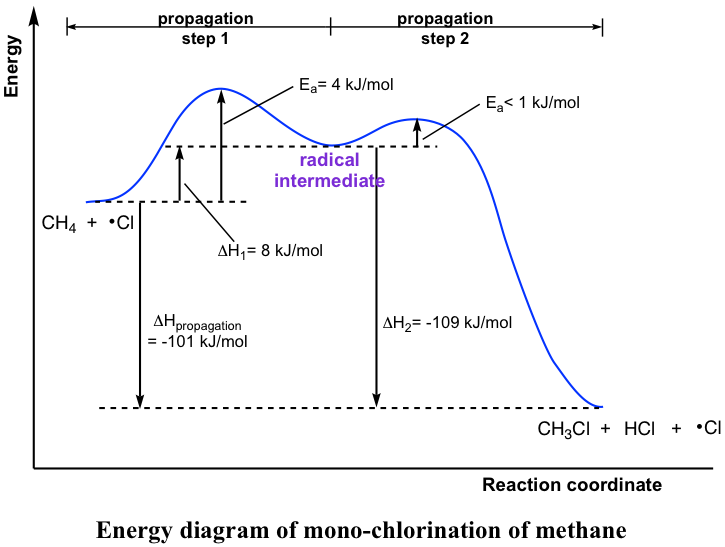
How to Draw Multi-Steps Energy Profile Diagrams: Reactant, Product, ∆H, Activation Energy, Slow Step
Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction.
CH 368: Unit 2 - University of Texas at Austin Conventionally, they are a plot of energy (in the rigorous case, free energy, G) versus the reaction coordinate. 6. Reaction Mechanism. The mechanism of a reaction is a stepwise description of how reactants are converted to products. Of course, some reactions occur in a single step, as represented in the reaction path diagram above. Such ...
Illustrating the overall reaction network of the synthesis ... Sep 15, 2022 · Typically, Fe-, Co-, Ni- and Ru-based materials are active FTS catalysts. 2, 3, 5 Owing to its compatibility for the water-gas shift reaction (WGSR: CO + H 2 O ⇌ H 2 + CO 2), iron-based FTS catalysts are currently the most pragmatic option, especially for feedstocks typically with a low H 2 /CO ratio (also an inexpensive and abundant option than other metals). 3, 10 There is an agreement ...
Acetyl-CoA carboxylase - Wikipedia Genes. The polypeptides composing the multi-subunit ACCs of prokaryotes and plants are encoded by distinct genes. In Escherichia coli, accA encodes the alpha subunit of the acetyl-CoA carboxylase, and accD encodes its beta subunit.. Mechanism. The overall reaction of ACAC(A,B) proceeds by a two-step mechanism. The first reaction is carried out by BC and …
Is the rate determining step the step with the largest Ea? 5. Yes, the rate determining step is the largest energy difference between any starting material or intermediate on a potential energy diagram and any transition state that comes after it. That transition state will then be the rate-determining step of a given reaction. The transition state with highest absolute energy may not necessarily ...
Analyzing Multi-step Reaction Energy Profiles - Study.com Analyzing Multi-step Reaction Energy Profiles High School Chemistry Skills Practice 1. Considering the graph, how many elementary steps are in the reaction mechanism? 2. On the following multi-step...
Analyzing Multi-step Reaction Energy Profiles | Chemistry - Study.com Step 1: Count the number of low points and high points in the diagram. Step 2: Correlate the low points to intermediates and the high points to transition ...
Acetyl-CoA carboxylase - Wikipedia The overall reaction of ACAC(A,B) proceeds by a two-step mechanism. The first reaction is carried out by BC and involves the ATP-dependent carboxylation of biotin with bicarbonate serving as the source of CO 2. The carboxyl group is transferred from biotin to acetyl CoA to form malonyl CoA in the second reaction, which is catalyzed by CT.
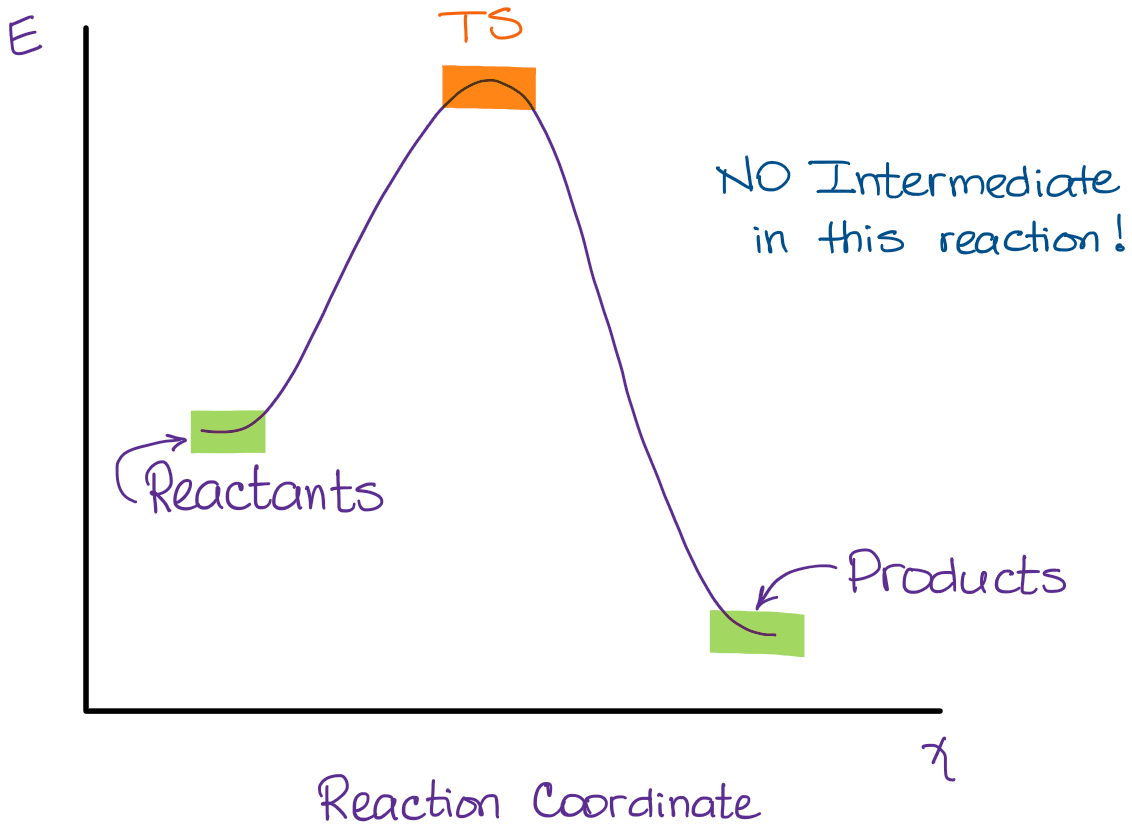
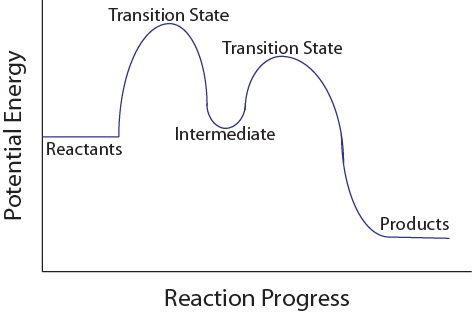
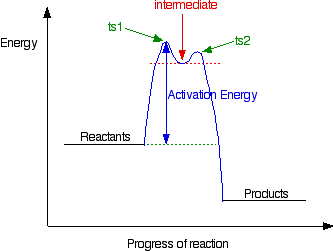
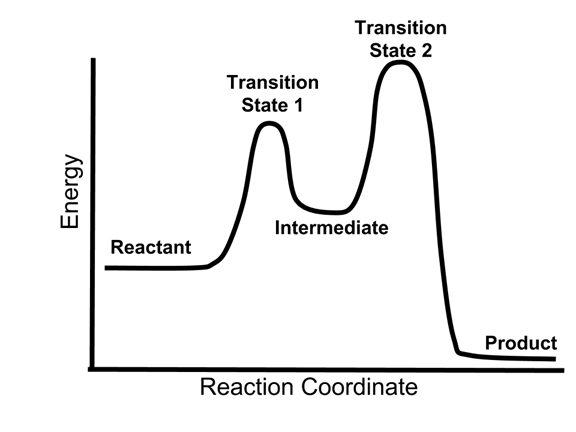
Energy Diagrams, Transition States, and Reactive Intermediates ... - JoVE In a multi-step reaction, each step has a transition state and corresponding activation energy. The transition states of such reactions are punctuated with reactive intermediates, which are represented as local minima on the energy diagrams. Reactive intermediates are products of bonds breaking and cannot be isolated for prolonged periods of time.
Multistep reaction energy profiles | Kinetics | AP Chemistry - YouTube Jan 30, 2021 ... Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used ...
How to Draw Multi-Steps Energy Profile Diagrams: Reactant ... - YouTube We'll go over how to label the reactants, products, transition state, activated complex, activation energy, ∆H delta H, forward reaction, reverse reaction. We'll go over how the activation energy...
Enzyme kinetics - Wikipedia The major contribution of the Henri-Michaelis-Menten approach was to think of enzyme reactions in two stages. In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis complex. The enzyme then catalyzes the chemical step in the reaction and releases the product.
Chem 51A. Lecture 19. Organic Chemistry: Ch. 6. Energy Diagrams ... -11:11 Energy Diagram for Single Step Reaction-17:13 Transition State-26:06 Energy Diagram for Multi-Step Reaction-34:46 Steps in the Multi-Step Reaction-30:01 Reverse Reaction of Multi-Step Reaction-41:34 Rates of Reaction-47:15 Other Factors Affecting Rate. Required attribution ...
Pseudo-adsorption and long-range redox coupling during oxygen … 01/04/2022 · Fig. 2: Energy diagram of different reaction intermediates of different transition metal single atom catalyst to form pseudo-adsorption state of OH δ−. The heatmap shows the Δ E to form each ...
Multistep reaction energy profiles | Kinetics | AP Chemistry | Khan ... Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the act...
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide Diagrams like this are described as energy profiles. In the diagram above, you can clearly see that you need an input of energy to get the reaction going. Once the activation energy barrier has been passed, you can also see that you get even more energy released, and so the reaction is overall exothermic. If you had an endothermic reaction, a ...
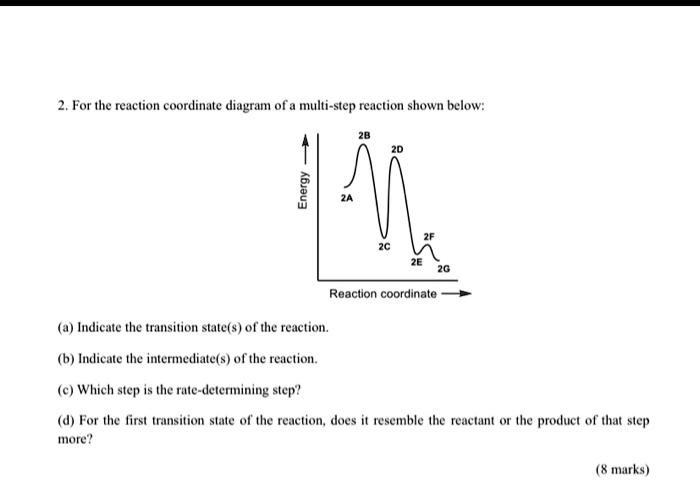
Solved 3. The energy diagram for a multi-step reaction - Chegg The energy diagram for a multi-step reaction mechanism for the decomposition of C4H9Br is shown below.
Pseudo-adsorption and long-range redox coupling ... - Nature Apr 01, 2022 · The energy diagram in Fig. 2 shows that it is rather thermodynamically unfavorable for the left *O species to undergo further protonation by the solvent water molecules for Fe-N 4 /C catalyst and ...
Apparent Activation Energies in Complex Reaction Mechanisms: A Simple ... This simplicity provides deep insight into the connection between the reaction energy diagram and the apparent activation energy. We prove both this and the quantitative validity of this equation by analysis of numerous reaction mechanisms. ... (DRC) is a mathematical approach for analysing multi-step reaction mechanisms that has proven very ...
Spin-polarized oxygen evolution reaction under magnetic field May 10, 2021 · The oxygen evolution reaction (OER) is the bottleneck that limits the energy efficiency of water-splitting. The process involves four electrons’ transfer and the generation of triplet state O2 ...
Spin-polarized oxygen evolution reaction under magnetic field 10/05/2021 · The oxygen evolution reaction (OER) is the bottleneck that limits the energy efficiency of water-splitting. The process involves four electrons’ transfer and the generation of triplet state O2 ...
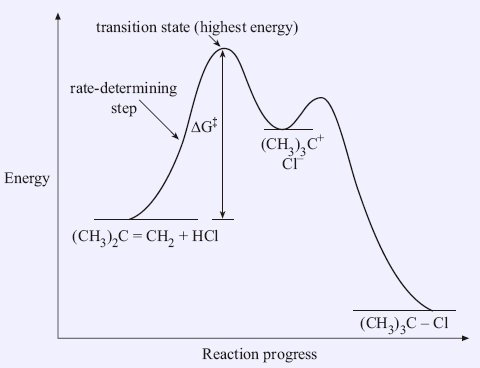
Energy Diagram for a Two-Step Reaction Mechanism Step 1 has the higher transition energy state, thus it is the rate-determining step. In many reactions more than one step is involved in the formation of products. Step Two Exothermic because energy is released in forming B-C bond delta H is negative Products are lower than the starting materials
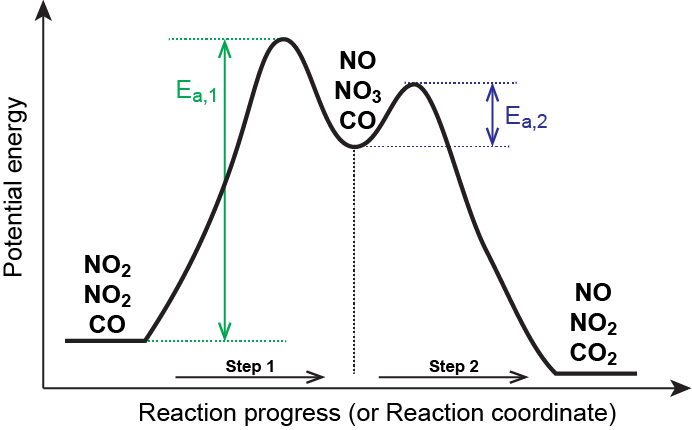
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
18.15: Mechanisms and Potential Energy Diagrams Figure 18.15. 1: The potential energy diagram shows an activation energy peak for each of the elementary steps of the reaction. The valley between represents the intermediate for the reaction. (CC BY-NC; CK-12) The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is ...
E1 Reaction Mechanism and E1 Practice Problems - Chemistry Steps Step 1: Loss of he leaving group. The energy diagram of the E1 mechanism demonstrates the loss of the leaving group as the slow step with the higher activation energy barrier: The dotted lines in the transition state indicate a partially broken C-Br bond. The Br being the more electronegative element is partially negatively charged and the ...
Reaction Mechanisms and Multistep Reactions - Chemistry LibreTexts Multi-step (Consecutive) Reactions Mechanisms in which one elementary step is followed by another are very common. A+ B → Q B+ Q → C A+ 2B → C (As must always be the case, the net reaction is just the sum of its elementary steps.) In this example, the species Q is an intermediate, usually an unstable or highly reactive species.
5.10 - Multistep Reaction Energy Profile - YouTube Nov 20, 2020 ... 5.10 - Multistep Reaction Energy Profile. Watch later. Share. Copy link. Info. Shopping. Tap to unmute. If playback doesn't begin shortly, ...
Enzyme kinetics - Wikipedia Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions.In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier …
Reaction mechanism and rate law (article) | Khan Academy A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step.




















Post a Comment for "44 multi step reaction energy diagram"